Which Law Is Described by the Equation P1v1 P2v2
Need help with chemistry. This answer has been confirmed as correct and helpful.
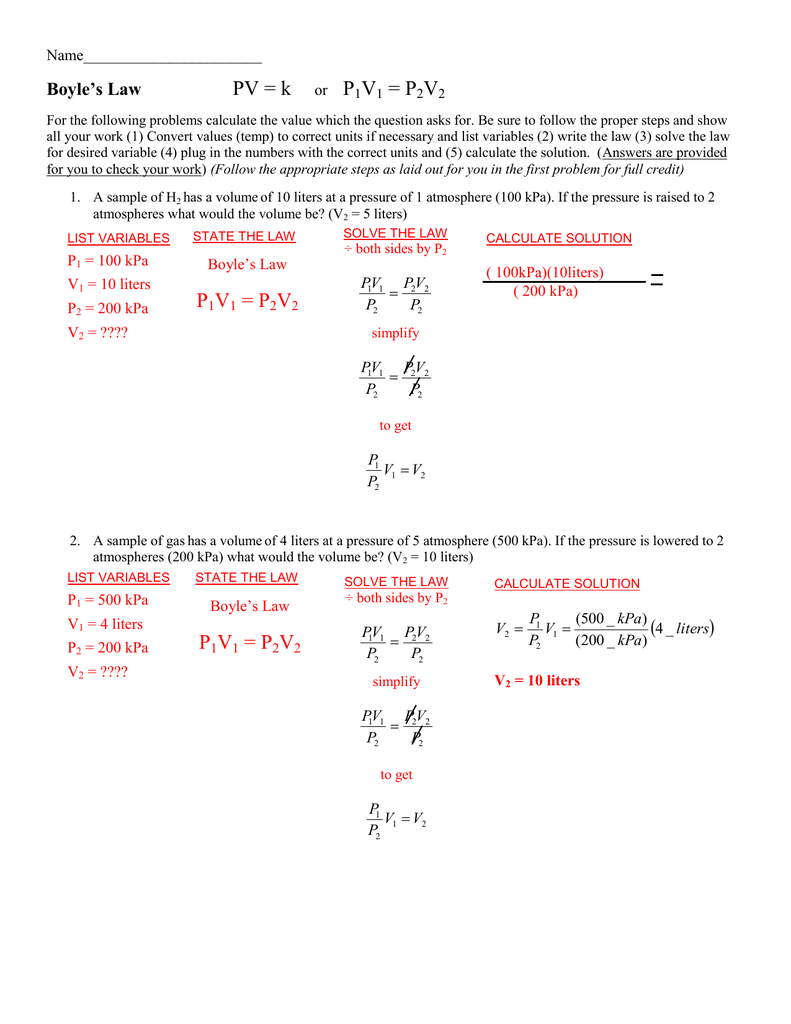
Pv K Or P1v1 P2v2 P1v1 P2v2 P1v1 P2v2
Boyles Law is described by the equation P1V1 P2V2.

. Boyles law states that pressure and volume have an inverse relationship. This problem has been solved. See the answer See the answer.
Boyles law states that the pressure of an ideal gas increases as its container volume decreases. Boyles Law assumes constant temperature P1V1 P2V2. See answer 1 Best Answer.
W I N D O W P A N E. P1V1T1 P2V2T2 Temperature must be in Kelvin and the pressure. P1V1 P2V2 Charles Law.
Sh hare your windo w. V2 1 bar x 227 L 02 bar 1135 L. Which law can be used to explain pressure volume temperature moles and universalideal gas constant as well as molar mass and mass.
If p1 is 1 bar V1 will be 227 L. If p2 02 bar then we have V2 p1V1p2. The correct option is P1V1 T1 P2V2 T2.
Added 1022018 84321 AM. This answer has been confirmed as correct and helpful. Confirmed by Sting 1022018 85037 AM 34872477.
Which simple gas law is represented by P1V1P2V2. Correct option is A Boyles law. Starting pressure times starting volume equals ending pressure.
Gay-Lussacs Law Charles Law Avogadros Law Boyles Law. V1 T1 V2 T2 GayLussacs. This problem has been solved.
In an equation form. Added 1022018 84911 AM. Be notified when an answer is posted.
Want this question answered. What law is described by the equation P1V1 P2V2. Simple steps and solved boyles law examples.
Boyles law states this fact. See the answerSee the answerSee the answerdone loading. The combine gas law states that the ratio of the product of pressure volume and absolute temperature of a gas is always a.
If you are doing an experiment to verify this law and you have a fixed amount of gas in a changeable volume when you change. This gas law allows you to do calculations for situations in which only the amount of gas is constant. Boyles Law is described by the equation P1V1 P2V2.
For a fixed amount of an ideal gas kept at a fixed temperature pressure and volume are inversely proportional. What law illustrated by the equation P1V1 equals P2V2. In this video we will do an example calculation using Boyles Law P1V1P2V2Solving for pressure P2.
From Boyles Law we have p1V1 p2V2. The Ideal Gas Law define the law described by the.

Boyle S Law Overview Formula Expii

Boyle S Law Practice Problems Examples Explained P1v1 P2v2 Youtube
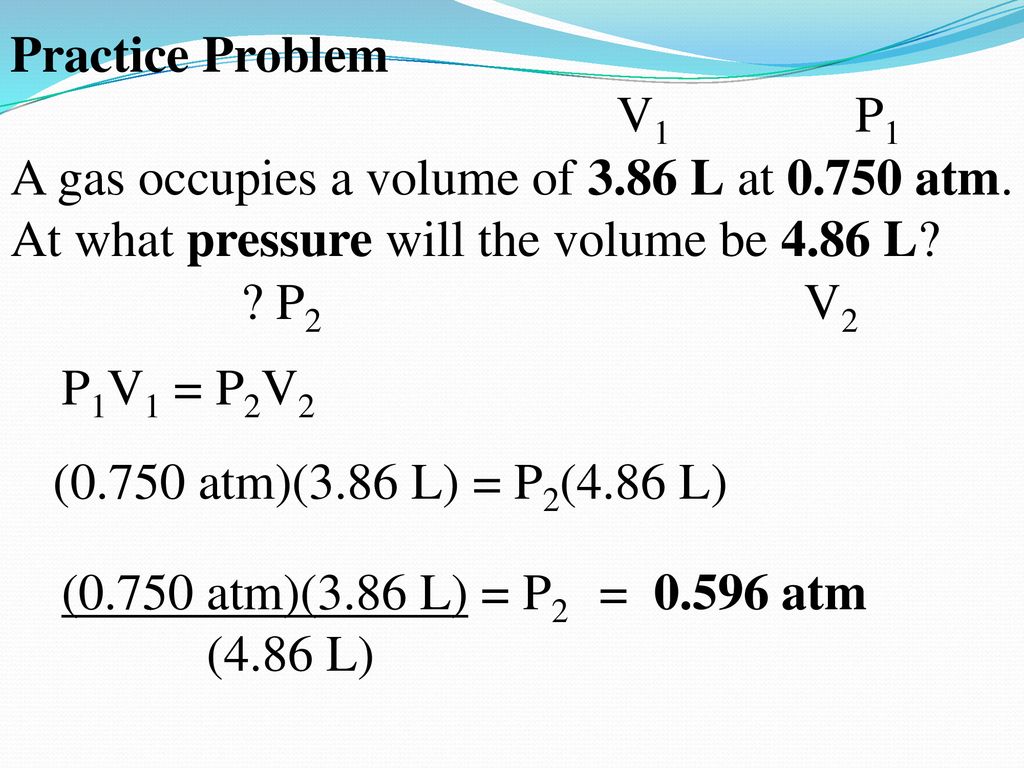
Boyle S Law Y A X Pressure A Volume Pv Constant P1v1 P2v2 Ppt Download
No comments for "Which Law Is Described by the Equation P1v1 P2v2"
Post a Comment